DUKORAL® demonstrated protective efficacy against LT-producing ETEC diarrhea and Cholera1
Efficacy of DUKORAL® against LT-producing ETEC diarrhea
Clinical trials were conducted with an earlier composition of DUKORAL®, formulated with purified native cholera toxin B subunit (CTB; referred to as BS in the clinical trials) and killed cholera whole cell extract (WC). The current composition of DUKORAL® contains recombinant cholera toxin B (rCTB), shown to be immunologically equivalent to native CTB. Two studies conducted in a total of 107 Swedish volunteers demonstrated comparable antitoxin and vibriocidal antibody titers after immunization with either rCTB or native CTB.1
VACCINE EFFICACY AGAINST EPISODES OF DIARRHEA CAUSED BY LT-PRODUCING ETEC1
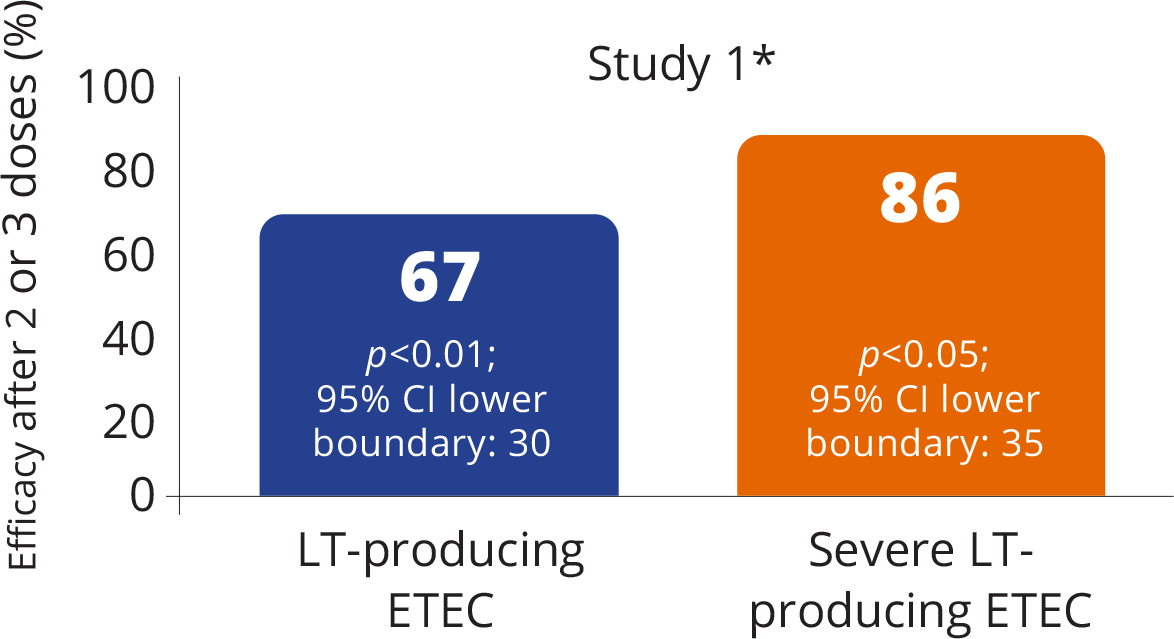
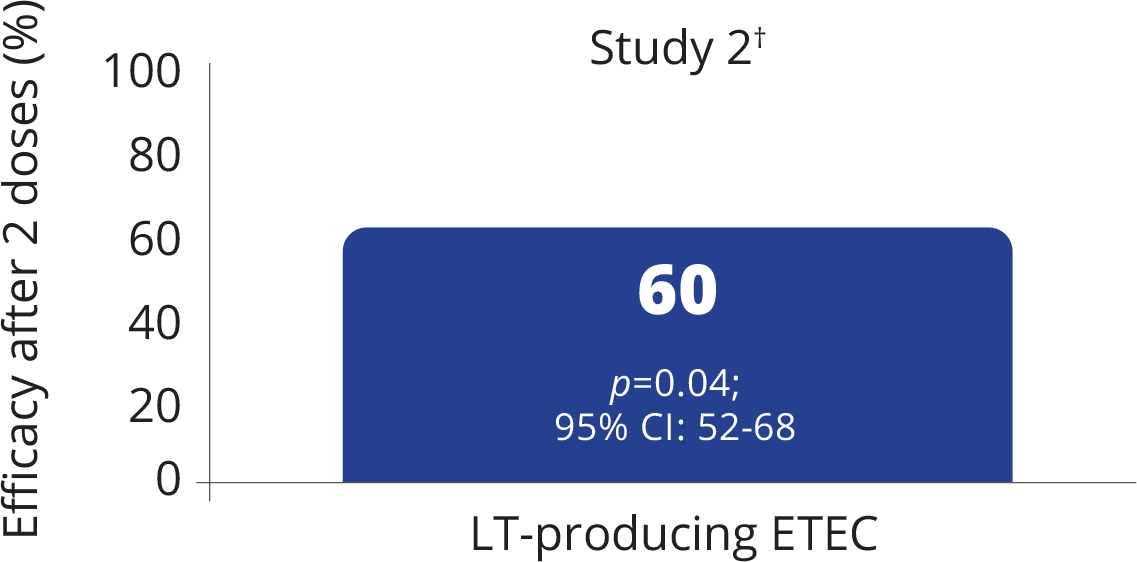
Adapted from the DUKORAL® Product Monograph.1
DUKORAL® had an 86% protective efficacy against clinically severe episodes of LT-producing ETEC1
Efficacy of DUKORAL® against cholera
Clinical trials were conducted with an earlier composition of DUKORAL®, formulated with purified native cholera toxin B subunit (CTB; referred to as BS in the clinical trials) and killed cholera whole cell extract (WC). The current composition of DUKORAL® contains recombinant cholera toxin B (rCTB), shown to be immunologically equivalent to native CTB. Two studies conducted in a total of 107 Swedish volunteers demonstrated comparable antitoxin and vibriocidal antibody titers after immunization with either rCTB or native CTB.1
VACCINE EFFICACY AGAINST CHOLERA1*
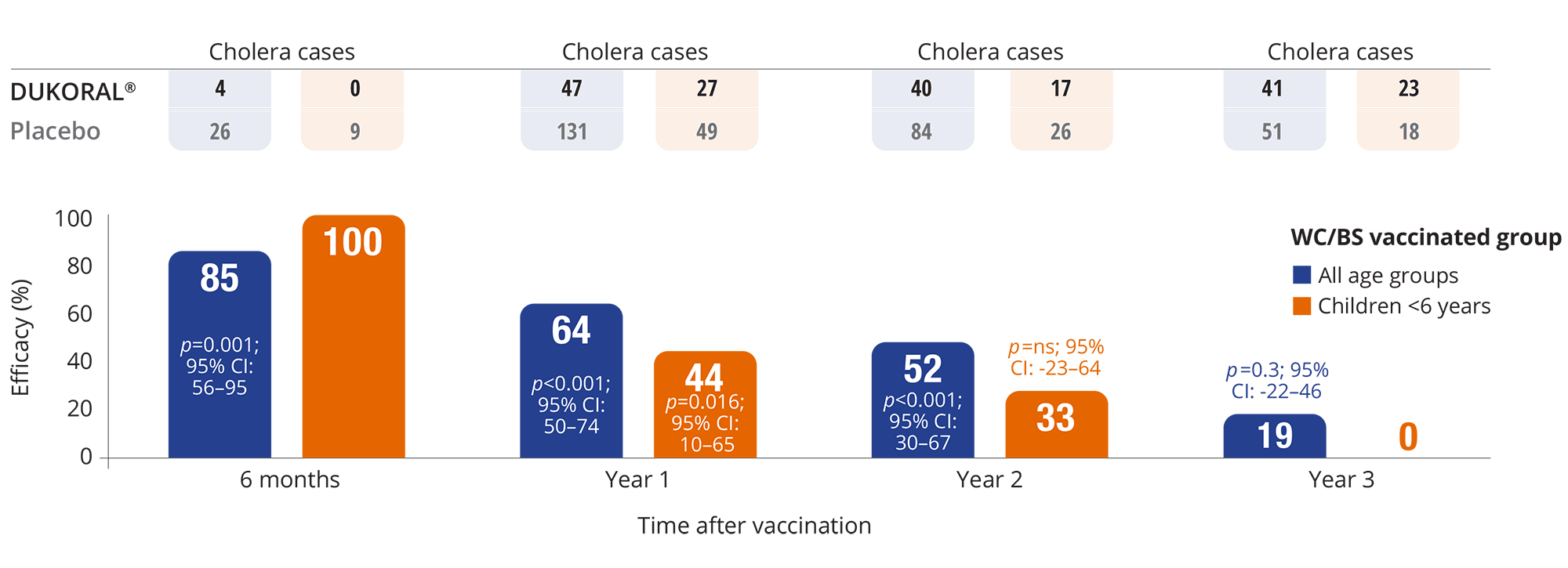
Adapted from the DUKORAL® Product Monograph.1
An exploratory analysis suggested that two vaccine doses seemed as effective as three doses in adults.1
Protective efficacy declined over the 3-year study period, declining more rapidly in those under 6 years of age.1
Help prevent cholera and LT-producing ETEC diarrhea with DUKORAL®
PROVEN SAFETY PROFILE1
In a clinical trial conducted in Bangladesh in adults and children above 2 years, 321 persons received 3 doses of DUKORAL® formulated with purified native cholera toxin B subunit (BS) and killed cholera whole cell extract (WC); and 323 persons received a control buffer without vaccine. Safety was assessed by active surveillance.
Adverse events reported following the first dose are shown in the table below. The frequency of adverse events was similar following subsequent doses. There were no significant differences between the groups. No serious adverse reactions were reported.
ADVERSE EVENTS REPORTED FOLLOWING FIRST DOSE IN ADULTS AND CHILDREN ABOVE 2 YEARS1
| Most frequently reported adverse events |
DUKORAL® n=321 |
Control n=323 |
| Gastrointestinal symptoms | ||
| Abdominal pain | 16% | 14% |
| Diarrhea | 12% | 11% |
| Nausea | 4% | 5% |
| Vomiting | 3% | 1% |
| General disorders and administration site conditions | ||
| Subjective fever | 4% | 5% |
| Other‡ | 1% | 1% |
Adapted from the DUKORAL® Product Monograph.1
Cholera: ns: not statistically significant
- Protective efficacy of DUKORAL® against cholera and diarrhea caused by LT-producing ETEC was evaluated in a randomized, double-blind, endemic field study performed in Bangladesh in adults and children aged 2 years and above. Efficacy data for LT-producing ETEC diarrhea was limited to the initial 3 months of follow-up with no protection demonstrated thereafter. 89,152 individuals received at least one dose of DUKORAL®, of whom 63,498 received three complete doses given at 6-week intervals. Subjects in each treatment group received either killed cholera whole cells (WC), with or without the purified native form of the cholera toxin B-subunit (BS) (n=21,141 and n=21,137, respectively), or placebo (n=21,220). Number of children <6 years in each treatment group were (WC/BS) 3,721, (WC) 3,871, and (placebo) 3,800.
- A prospective double-blind trial investigating vaccine efficacy after 2 doses against episodes of diarrhea caused by different types of ETEC in 615 healthy Finnish tourists travelling to Morocco aged 15 years and older. Each patient received 2 doses of either DUKORAL® (n=307) or placebo (n=308) before the trip departure.
- ‡ Symptoms requiring bedrest. Complaints included headache and myalgias, generalized weakness and faintness, headache and coryza and generalized weakness.


